Sweeteners

Although, using natural sugar to excess has its own set of health problems, natural sugar used in moderation is still a healthier sweetener choice than sucralose
Sucralose is NOT a sugar, despite its sugar-like name and deceptive marketing slogan - it is, in fact, a chlorinated artificial sweetener with detrimental health effects to match aspartame
Sucralose® (trade name Splenda or E.U. additive code E955) is the #1 artificial sweetener in the U.S. - generally used as a sugar substitute
Sucralose is a synthetic chemical made by a patented process by McNeil Nutritionals that does indeed begin with sugar(sucrose, a disaccharide molecule about 50/50 glucose and fructose). Three hydroxyl (OH) groups are replaced with 3 chlorine molecules, producing a fructose + galactose molecule not seen in nature.
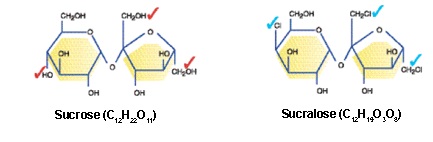
Image from Splenda-Clinician's Guide @ www.splenda.com
Ahhhh. . . just the way grandma used to make it!
http://roarofwolverine.com/archives/185
The end product is a chlorinated hydrocarbon molecule (a.k.a. an organochlorine or chlorocarbon) - i.e. NOT a sugar molecule.
Sucralose was actually discovered by accident by Tate and Lyle scientists working with researchers at QueenElizabethCollege, trying to create new insecticides. it was discovered by Leslie Hough and a young Indian chemist, Shashikant Phadnis as the duo was trying to test chlorinated sugars as chemical intermediates.
Phadnis was told to test the powder, but thought that Hough had asked him to taste it. He found the compound to be exceptionally sweet. After this revelation, they worked with Tate & Lyle for a year before settling on the final formula.
In 1980 the rights of sucralose were sold to Johnson and Johnson who then created McNeil Nutritionals to be solely responsible for the marketing of Splenda®. Later in 2004, McNeil Nutritionals and Tate & Lyle restructured their alliance so that McNeil was responsible for marketing and Tate & Lyle for manufacturing the product.
In 1989, sucralose was approved for use in the United States and Diet R.C. Cola was the first product to contain it
"Sucralose" is a cute short name for:
1,6-dichloro-1,6-dideoxy-BETA-D-fructofuranosyl-4-chloro-4-deoxy-alpha-D-galactopyranoside
Chlorine in sucralose is not the same safe form as covalent chloride bonds in food. Actually, nature contains NO covalent chloride-to-organic compound bonds.
Examples of other synthetic organochlorines (long known for causing organ, genetic and reproductive damage) include:
J & J maintain that sucralose chlorocarbons are not a problem since sucralose is not absorbed -however, the fact is that the final rule of the FDA was that a significant percentage actually is absorbed in the body.
Organochlorines don't breakdown easily in fatty tissue and can build up over time - in his book "Sweet Deception", Dr. Joseph Mercola details how researchersfound evidence that Splenda is in fact absorbed by your fat and tends to accumulate in high-fat organ tissues (E.g. your brain) over time.
Researcher / biochemist Dr. James Bowen states that ingested chlorocarbon damage continues with the formation of other toxins:"Any chlorocarbons not directly excreted from the body intact can cause immense damage to the processes of human metabolism and, eventually, our internal organs. The liver is a detoxification organ which deals with ingested poisons. Chlorocarbons damage the hepatocytes, the liver's metabolic cells, and destroy them. In test animals, Splenda®produced swollen livers, as do all chlorocarbon poisons, and also calcified the kidneys of test animals in toxicity studies. The brain and nervous system are highly subject to metabolic toxicities and solvency damage by these chemicals. Their high solvency attacks the human nervous system and many other body systems including genetics and the immune function. Thus, chlorocarbon poisoning can cause cancer, birth defects, and immune system destruction. These are well known effects of Dioxin and PCBs which are known deadly chlorocarbons."
Dr. James Bowen, Article:"The Lethal Science Of Splenda - A Poisonous Chlorocarbon"
Ingested sucralose breaks down into products (1,6 dichloro, 1,6-dideoxyfructose,4-chloro-4-deoxygalactose and potentially highly toxic chlorosugar 6-GC) and has been proven in tests to to be able to have the following adverse effects:
McNeil claims that Splenda® has zero calories since it is not absorbed by the body - because the body has no enzymes to break down /digest this unnatural, glucose-free molecule, however . . .
The FDA's "Final Rule" reported 11% to 27% of sucralose is absorbed in humans:
Michael A. Friedman, Lead Deputy Commissioner for the FDA,Food Additives Permitted for Direct Addition to Food for Human Consumption; SucraloseFederal Register: 21 CFR Part 172, Docket No. 87F-0086, April 3, 1998
The Japanese Food Sanitation Council reports that up to 40% of ingested sucralose is absorbed - and can concentrate in the liver, kidney, and GI tract
The FDA allowed sucralose absorption / metabolism findings of just ONE 8 man study to be generalized to the entire population - women, children, the elderly, and those with any chronic illness were never examined.
Roberts A, Renwick AG, Sims J, Snodin DJ. Sucralose metabolism and pharmacokinetics in man. Food Chem Toxicol. 2000;38 Suppl 2:S31-41. PubMed
Splenda has NEVER been proven safe for HUMAN consumption!
|
The marketing pitch for Spenda emphasizes that it has undergone rigorous testing, but fail to mention that nearly all tests were on animals (initial studies showing health detrimental results) and only 2 small (almost laughable) studies lasting less than 4 days on humans prior to FDA approval |
Initially, the EU Food Commision, Canadian officials and the U.S. FDA did NOT approve Splenda, based on several serious health problems revealed in animals -so McNeil Nutritionals (Sucralose manufacturer, a subsidiary of Johnson and Johnson) continued their research studies, lowering levels of sucralose administered until favorable results were obtained. Of course, the negative research results were not mentioned.
Splenda®/Sucralose was given the broadest approval ever granted by the FDA for any food additive based on the review of of 108 animal studies and only two human studies lasting only a few days - in 1998 it was approved for use in 15 food and beverage categories, with no requirement for warnings or informational labels on products containing sucralose. A year or so later, the FDA approved sucralose as a general-purpose sweetener.
Of those 2 human studies:
The animal studies reviewed revealed several problems:
A 2008 Duke University study found that FDA-approved food levels of Splenda:
Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, McLendon RE, Schiffman SS. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome p-450 in male rats. J Toxicol Environ Health A. 2008;71(21):1415-29. Researchgate
"Increasing evidence suggests that artificial sweeteners do not activate the food reward pathways in the same fashion as natural sweeteners - Lack of caloric contribution generally eliminates the postingestive component. Functional magnetic imaging in normal weight men showed that glucose ingestion resulted in a prolonged signal depression in the hypothalamus. This response was not observed with sucralose ingestion."
Smeets PAM, de Graaf C, Stafleu A, van Osch MJP, van der Grond J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am J Clin Nutr. 2005;82:1011-1016. PubMed
The following are common symptoms - usually noticed within a 24-hour period following consumption of Splenda products:
Many people have found that it is easier to lose weight by cutting out sweets altogether instead of just replacing the natural sugar with an artificial one
NNS - May not be such a sweet deal
Natural sweeteners in moderation should be preferred to any artificial sweeteners